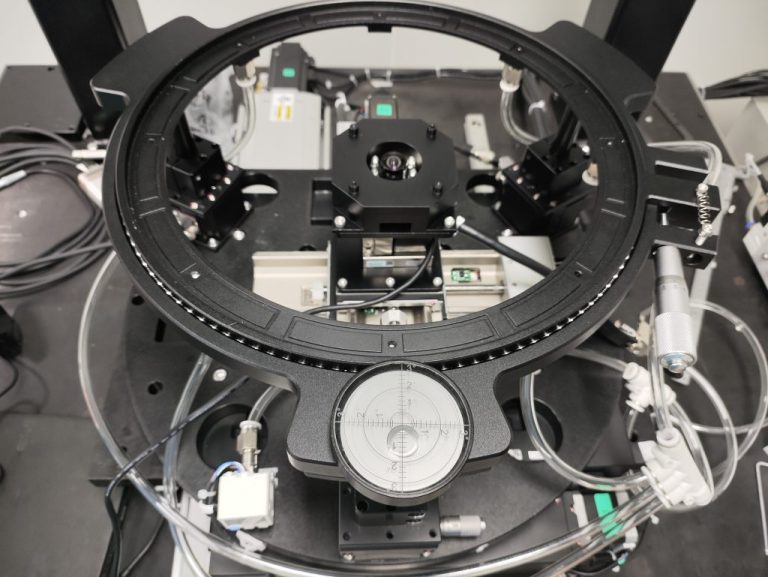
Product Introduction
The uterine ablation system is a Class III medical device designed for the treatment of gynecological conditions, with stringent requirements for safety, reliability, and compliance.
Project Overview
This project was initiated by an experienced medical device entrepreneur from the San Francisco Bay Area, reflecting the demand for cutting-edge medical technologies. Introduced through Bay Area partners, TronixV was entrusted with the complete development and production support of the device.
Product Development
As a Class III device, the project required full CFDA certification compliance, including reliability, EMC, and electrical safety testing, along with ISO13485-compliant documentation throughout development. Over an 18-month period, TronixV successfully delivered the device, supporting all necessary regulatory requirements and enabling the client to obtain CFDA approval.
Manufacturing
TronixV handled prototype manufacturing and customer validation. For mass production, the project was transferred to a certified ISO13485 factory, while TronixV continued to provide technical support and iterative improvements to ensure long-term product reliability.