The medical device industry stands at the forefront of healthcare innovation, with research and development (R&D) serving as the driving force behind life-saving technologies. From diagnostic equipment to surgical instruments, medical device R&D transforms scientific discoveries into practical solutions that improve patient outcomes and revolutionize healthcare delivery. Understanding the intricacies of this field is crucial for healthcare professionals, entrepreneurs, and anyone interested in the intersection of technology and medicine.
What is Medical Device R&D?
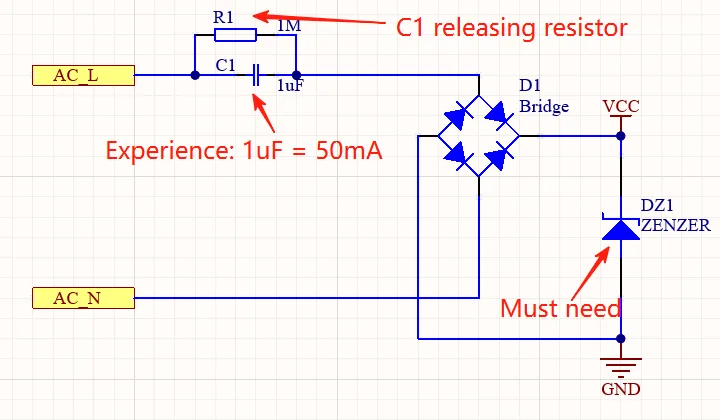
Medical device research and development encompasses the systematic process of creating, testing, and refining healthcare technologies that diagnose, treat, monitor, or prevent medical conditions. This comprehensive field combines scientific research, engineering innovation, and regulatory compliance to bring safe and effective medical devices to market.
Medical device R&D operates within a highly regulated environment, requiring adherence to stringent safety standards and regulatory requirements established by organizations such as the FDA, CE marking authorities, and other international regulatory bodies. The process typically spans several years and involves multiple phases, from initial concept development through clinical trials and market approval.
The scope of medical device R&D is remarkably broad, covering everything from simple diagnostic tools to complex implantable devices. This includes cardiovascular devices, orthopedic implants, diagnostic imaging equipment, surgical instruments, patient monitoring systems, and emerging technologies like artificial intelligence-powered diagnostic tools and robotic surgical systems.
What is R&D in Medical Terms?
In medical terminology, R&D refers to the structured approach of advancing medical knowledge and translating scientific discoveries into practical healthcare applications. Medical R&D encompasses both basic research, which seeks to understand fundamental biological processes and disease mechanisms, and applied research, which focuses on developing specific medical interventions and technologies.
The medical R&D process follows established scientific methodologies, including hypothesis formation, experimental design, data collection and analysis, peer review, and replication studies. This rigorous approach ensures that medical innovations are based on solid scientific evidence and meet the highest standards of safety and efficacy.
Medical R&D also involves translational research, which bridges the gap between laboratory discoveries and clinical applications. This critical phase transforms promising research findings into viable medical treatments, devices, or diagnostic tools that can be safely and effectively used in healthcare settings.
What Does Someone in R&D Do?
Professionals working in medical device R&D engage in diverse activities that span the entire product development lifecycle. Their responsibilities typically include conducting literature reviews and market research to identify unmet medical needs and emerging opportunities, designing and executing experiments to test new concepts and technologies, and collaborating with clinical professionals to understand real-world healthcare challenges.
R&D professionals also play crucial roles in prototype development and testing, working with engineering teams to create functional models of new medical devices. They conduct extensive testing to evaluate device performance, safety, and reliability under various conditions, ensuring compliance with regulatory standards and industry best practices.
Documentation and reporting represent another significant aspect of R&D work. Professionals must maintain detailed records of research activities, compile technical reports, and prepare regulatory submissions. They also participate in patent applications, scientific publications, and conference presentations to share their findings with the broader medical community.
Collaboration is essential in medical device R&D, as professionals work closely with cross-functional teams including engineers, clinicians, regulatory affairs specialists, quality assurance experts, and marketing professionals. This collaborative approach ensures that new medical devices meet both technical specifications and practical healthcare needs.
What Does R&D Mean in Medical Terms?
R&D in medical terms represents the continuous cycle of innovation that drives healthcare advancement. It encompasses the systematic investigation of medical problems, the development of innovative solutions, and the rigorous testing required to ensure patient safety and treatment efficacy.
Medical R&D follows evidence-based principles, requiring substantial proof of concept, safety data, and clinical evidence before new technologies can be implemented in healthcare settings. This process includes preclinical studies, which evaluate device performance and safety in laboratory and animal models, followed by clinical trials that assess effectiveness and safety in human subjects.
The regulatory aspect of medical R&D is particularly significant, as new medical devices must receive approval from relevant regulatory authorities before commercialization. This requires comprehensive documentation demonstrating device safety, efficacy, and manufacturing quality, along with post-market surveillance to monitor long-term performance and safety.
Why R&D is Vital for Medical Device Companies
Medical device R&D serves as the cornerstone of innovation and competitive advantage in the healthcare technology industry. Companies that invest in robust R&D programs are better positioned to address evolving healthcare needs, comply with changing regulatory requirements, and maintain market leadership in their respective segments.
R&D enables medical device companies to develop breakthrough technologies that can transform patient care and create new market opportunities. By investing in research and development, companies can identify emerging trends, anticipate future healthcare needs, and develop solutions that address unmet medical challenges.
The competitive landscape in medical devices is intensely innovation-driven, making R&D investment essential for long-term success. Companies with strong R&D capabilities can differentiate their products, command premium pricing, and build sustainable competitive advantages that are difficult for competitors to replicate.
R&D also plays a crucial role in risk mitigation and regulatory compliance. Through thorough research and testing, companies can identify potential safety issues early in the development process, reducing the risk of costly recalls or regulatory setbacks. This proactive approach to quality and safety builds trust with healthcare professionals, patients, and regulatory authorities.
For companies seeking to excel in medical device development, partnering with specialized R&D service providers can offer significant advantages. TronixV’s Medical Device R&D Service provides comprehensive support throughout the development lifecycle, from initial concept development through regulatory approval and commercialization. Their experienced team of engineers, scientists, and regulatory experts can help accelerate time-to-market while ensuring compliance with all relevant safety and quality standards. TronixV’s proven track record in medical device development makes them an ideal partner for companies looking to innovate and succeed in the competitive healthcare technology market.
Conclusion
Medical device R&D represents a critical investment in the future of healthcare, driving innovation that saves lives and improves patient outcomes worldwide. The complex process of developing medical devices requires specialized expertise, substantial resources, and unwavering commitment to safety and quality. As healthcare needs continue to evolve and technology advances at an unprecedented pace, the importance of effective medical device R&D will only continue to grow.
Success in medical device development requires not only technical expertise but also deep understanding of regulatory requirements, clinical needs, and market dynamics. Companies that recognize the strategic importance of R&D and invest accordingly will be best positioned to lead the next generation of healthcare innovation and make meaningful contributions to global health improvement.
In the actual process of R&D, different projects often encounter various challenges in functional validation, structural design, component selection, and adaptation to clinical requirements. If you wish to reference more real-world projects, we have also compiled several actual development cases, which provide insights into the complete R&D process and implementation methods.
If you are seeking professional assistance to advance design, electronic development, or full-scale production, feel free to contact our engineering team anytime for specialized technical support and tailored solutions.