Project Background: Urgent Need for Cutting-Edge Medical Technology R&D and Powerful Alliance
Limb orthopedic and lengthening technology has gone through decades of development, from the classic Ilizarov external fixation technology to the new generation of in vitro controlled, fully implantable intramedullary lengthening nails, achieving a leap from “invasive and inefficient” to “minimally invasive and precise”.
However, referring to international advanced experience, the domestic independent research and development of equipment and clinical applications in this field are still at a blank stage, and it is urgent to break through the core technology bottleneck and fill the market gap.
Against this background, TronixV and Tsinghua University have carried out in-depth cooperation, relying on Tsinghua University’s cutting-edge research results in the fields of biomedical engineering and precision machinery, combined with Tongwei Technology’s mature experience in the entire chain of “R&D-Production-Compliance” of medical devices, to jointly promote the research and development and industrialization of the in vitro controlled intramedullary lengthening nail drive control host.
The project aims to solve the problems of high infection risk, poor patient comfort, and insufficient lengthening accuracy in traditional limb lengthening technology, and help domestic limb orthopedic technology to keep pace with international standards.

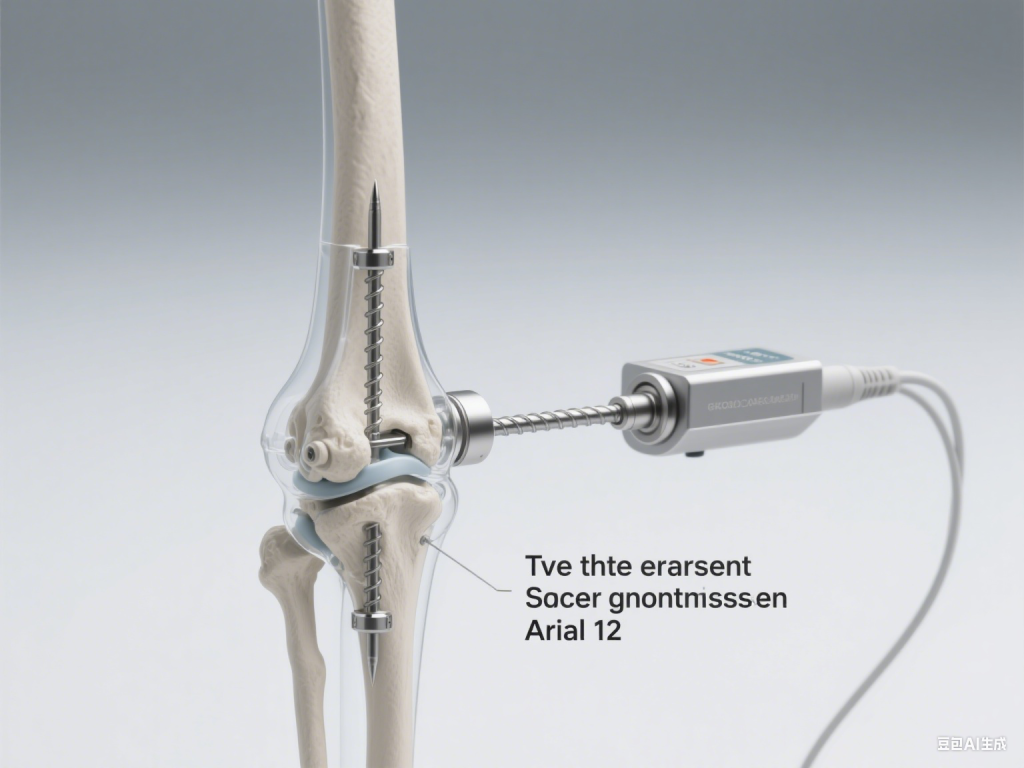
Technical solution: With a three-phase motor drive as the core, create a precise and controllable extension mechanism
The core requirements of the in vitro controlled intramedullary lengthening nail are “precise drive, slow and stable, safe and reliable”. After implantation, the drive system needs to achieve high-precision extension of 0.1-1mm/d through in vitro control, and complications such as “uncontrolled extension” need to be avoided. Based on this, TronixV proposed a technical solution based on a three-phase motor drive:
Innovative application of the three-phase motor drive principle
A high-performance three-phase permanent magnet synchronous motor is used as the power core, and low-speed and high-torque output is achieved through a vector control algorithm. Compared with traditional mechanical ratchet drive or early electric drive systems, the three-phase motor has better stability when running at low speed, and can accurately control the speed as low as 0.01r/min, ensuring that the intramedullary nail extension accuracy reaches 0.01mm level, which perfectly matches the clinical demand of “daily staged micro-extension”.
Closed-loop feedback and an intelligent control algorithm
The system integrates high-precision displacement sensors and torque monitoring modules to provide real-time feedback on extension length and resistance changes. The motor output is dynamically adjusted through the PID algorithm to avoid extension speed fluctuations caused by changes in bone resistance. At the same time, multiple safety redundancy mechanisms are designed to automatically trigger shutdown protection when abnormal torque or speed is detected, eliminating the risk of “uncontrolled extension”.
Miniaturization and biocompatibility design
In view of the space limitations of implantable devices, TronixV has miniaturized the drive module, controlled the motor diameter within 10mm, and the overall structure conforms to the anatomical characteristics of human bones. The shell is made of medical-grade titanium alloy material and has passed the biocompatibility test to ensure the safety of long-term implantation.
Project achievements and value: efficient implementation and accelerated clinical transformation
Rapidly achieve the leap from R&D to mass production
Relying on TronixV’s mature R&D system and production capabilities, the project team completed the iteration from technical prototype to mass production sample in just 12 months, which is 40% shorter than the industry average cycle. Through modular design and automated production line deployment, the stable mass production of the drive host is achieved, with a yield rate of 99.5%.
Assisting customers to pass CFDA type inspection
TronixV participated in the product compliance design throughout the process. According to the “Medical Device Supervision and Administration Regulations” and relevant standards for orthopedic implants, it assisted customers in completing 23 testing items, such as biocompatibility, electrical safety, and mechanical properties, and passed the CFDA type inspection at one time, paving the way for the product to be launched on the market.
Promoting the upgrading of domestic limb orthopedic technology
The successful development of the drive control host has broken the monopoly of foreign brands in the field of in vitro controlled intramedullary lengthening nails, allowing domestic patients to enjoy safer and more comfortable limb lengthening treatment. At present, the intramedullary nails equipped with this system have entered the clinical trial stage and are expected to benefit patients with congenital limb deformities, post-traumatic limb shortening, etc.




